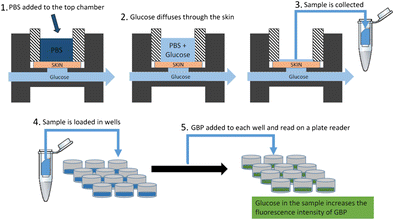
Current glucose monitoring techniques for neonates rely heavily on blood glucose monitors which require intermittent blood collection through skin-penetrating pricks on the heel or fingers. This procedure is painful and often not clinically conducive, which presents a need for a noninvasive method for monitoring glucose in neonates. Our motivation for this study was to develop an in vitro method for measuring passive diffusion of glucose in premature neonatal skin using a porcine skin model. Such a model will allow us to initially test new devices for noninvasive glucose monitoring without having to do in vivo testing of newborns. The in vitro model is demonstrated by comparing uncompromised and tape-stripped skin in an in-line flow-through diffusion apparatus with glucose concentrations that mimic the hypo-, normo-, and hyper-glycemic conditions in the neonate (2.0, 5.0, and 20 mM, respectively). Transepidermal water loss (TEWL) of the tape-stripped skin was approximately 20 g m-2 h-1, which closely mimics TEWL for neonatal skin at about 190 days post-conceptional age. The tape-stripped skin showed a >15-fold increase in glucose diffusion compared to the uncompromised skin. The very small concentrations of collected glucose were measured with a highly selective and highly sensitive fluorescent glucose biosensor based on the glucose binding protein (GBP). The demonstrated method of glucose determination is noninvasive and painless, which makes it especially desirable for glucose testing in neonates and children. This study is an important step towards an in vitro model for noninvasive real-time glucose monitoring that may be easily transferred to the clinic for glucose monitoring in neonates. Graphical Abstract Glucose diffusion through model skin was measured using an in-line flow-through diffusion apparatus with glucose solutions mimicking hypo-, normo- and hyperglycemia in the neonate. Phosphate buffered saline was added to the top chamber and the glucose that diffused through the model skin into the buffer was measured using a fluorescent glucose binding protein biosensor.
Tiangco, C., Andar, A., Quarterman, J. et al. Anal Bioanal Chem (2017) 409: 3475. https://doi.org/10.1007/s00216-017-0289-7